Did you know that your small intestine stretches about 6-7 meters inside your abdomen?
The small intestine’s histology shows an intricate design that optimizes nutrient absorption and digestion.
A microscopic analysis reveals three distinct parts of the small intestine: the duodenum, jejunum, and ileum. Each part has its own specialized tissues and cellular arrangements. The small intestine’s tissue has remarkable finger-like projections called villi that substantially increase the absorptive area[-5]. These villi create a distinctive “hills and valleys” appearance that makes the small intestine’s epithelium unique.
This piece explains the histological layers of the small intestine and shows you the various cell types that make up this organ. You’ll understand the regional differences between its three sections. It also helps you see how changes in small intestine histology connect to various clinical conditions, linking microscopic structure to physiological function and disease.
Anatomy and Basic Structure of the Small Intestine
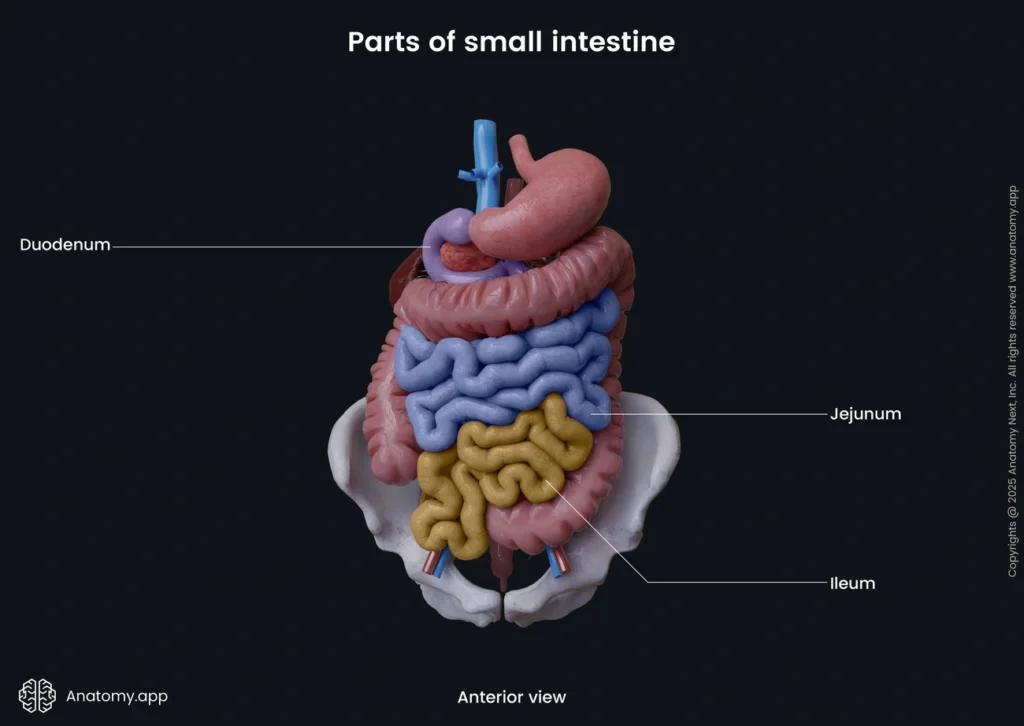
The small intestine is your body’s main site for nutrient digestion and absorption. This vital organ is surprisingly long and measures between 3-5 meters in adults, with some sources noting lengths up to 6.5 meters.
Parts of the small intestine: duodenum, jejunum, ileum
Your small intestine has three distinct segments that each serve specific functions:
The duodenum is the first, widest, and shortest section that extends about 20-25 cm from the stomach’s pylorus. This C-shaped tube wraps around your pancreas’s head and has four parts: superior, descending, horizontal, and ascending. The duodenum gets partially digested food (chyme) from the stomach and features the ampulla of Vater, where bile and pancreatic ducts release their contents to help digestion.
The jejunum makes up the middle segment and stretches roughly 2.5 meters. You’ll find it mainly in your upper left abdomen. The jejunum has a deeper red color, thicker walls, and better blood supply than the ileum. It makes up about two-fifths of the jejunum-ileum continuum.
The ileum is the final and longest segment at about 3 meters. It sits mainly in your lower right abdomen. The ileum connects to the large intestine at the ileocecal valve. Unlike the jejunum, it has more lymphoid tissue (Peyer’s patches) and mesenteric fat.
Length, location, and connection to other organs
The small intestine sits strategically in your abdominal cavity. While the duodenum starts at the stomach’s pyloric sphincter and stays mostly behind the peritoneum, the jejunum and ileum float freely, attached to the back abdominal wall by mesentery.
This coiled tube fills much of your abdominal cavity. It connects to several key organs:
- The stomach joins at the pyloric sphincter
- The pancreas and liver send digestive enzymes and bile to the duodenum
- The large intestine connects at the ileocecal valve
Blood flows mainly from the superior mesenteric artery, while the duodenum gets additional supply from the celiac trunk. Your intestinal wall’s enteric nervous system coordinates muscle activity to move contents through.
Role in digestion and absorption
The small intestine is vital for both digestion and absorption:
Digestive functions: Your small intestine breaks down food by:
- Mixing food with digestive juices from the pancreas, liver, and intestine
- Making enzymes that break down proteins, carbohydrates, and fats
- Using bacteria that produce enzymes needed for carbohydrate digestion
Absorption functions: The small intestine absorbs about 90% of nutrients efficiently. In healthy adults, it processes more than 95% of ingested carbohydrates and proteins. Each part specializes in different nutrients:
- The duodenum handles iron absorption and starts nutrient processing
- The jejunum takes in carbohydrates, fats, minerals, proteins, and vitamins
- The ileum processes vitamin B12, bile salts, and remaining nutrients
The small intestine also absorbs 7 of the 9 liters of fluid that enters it daily. Only 1.5-2 liters move on to the large intestine.
Histological Layers of the Small Intestine
The small intestine’s histology reveals four distinct layers under a microscope. These layers work together and support both digestive and absorptive functions. Each layer contains specialized structures that make this vital organ remarkably efficient.
Mucosa: villi, crypts, and epithelium
The innermost layer of small intestine tissue is the mucosa. It has three components: epithelium, lamina propria, and muscularis mucosae. The small intestine’s epithelium stands out because of its extensive surface modifications. The mucosa creates finger-like projections called villi that extend 0.5-1.5 mm into the lumen. These villi increase the absorptive surface area dramatically.
Simple columnar epithelial cells known as enterocytes line these villi. The enterocytes have microvilli on their apical surface, which we call the brush border. This increases the absorptive area even more. You’ll find several specialized cells scattered among the enterocytes:
- Goblet cells – Secrete protective mucus that lubricates the intestinal lining
- Paneth cells – Located at the base of crypts, they secrete antimicrobial peptides including defensins and lysozyme
- Enteroendocrine cells – Release hormones that regulate digestion and motility
- Tuft cells – Sense luminal contents and help regulate immune responses
The crypts of Lieberkühn lie between the villi. These tube-like invaginations contain stem cells that renew the epithelium every 3-5 days. The rapid turnover lets these cells repair quickly. The downside is that they become sensitive to radiation therapy and chemotherapy.
Submucosa: connective tissue and Brunner’s glands
The submucosa sits beneath the mucosa. This connective tissue layer contains blood vessels, lymphatics, and the submucosal (Meissner’s) nerve plexus. The plexus controls secretions and local blood flow, so it plays a vital role in the small intestine’s function.
Brunner’s glands appear in the duodenum’s submucosa and secrete alkaline mucus. These compound, tubular mucous glands cluster near the pylorus. They become shorter and fewer toward the distal duodenum. Their main goal is to neutralize acidic chyme from the stomach. This creates the right environment for intestinal enzymes to work. These glands fill the duodenal submucosa so completely that you can barely see the typical submucosal connective tissue.
Muscularis externa and Auerbach’s plexus
The muscularis externa (or muscularis propria) has two smooth muscle layers. The inner circular layer and outer longitudinal layer create peristaltic movements that push food through the digestive tract.
The myenteric (Auerbach’s) plexus sits between these muscle layers. This network of ganglia and nerve fibers controls gut motility. Leopold Auerbach (1828-1897) gave his name to this plexus. It works on its own but also receives signals from the autonomic nervous system.
The Auerbach plexus contains both activating cholinergic neurons that use acetylcholine and inhibitory nitrogenergic neurons that use nitric oxide. These neurons coordinate with interstitial cells of Cajal. These cells act as the gut’s electrical pacemakers and help coordinate rhythmic peristaltic contractions.
Serosa and mesentery
The serosa forms the small intestine’s outermost layer. This thin membrane consists of mesothelium and epithelium. It covers the jejunum, ileum, and only the intraperitoneal parts of the duodenum. Some duodenal parts remain retroperitoneal.
The mesentery anchors the jejunum and ileum to the posterior abdominal wall. It’s made of a double layer of peritoneum. The mesentery does more than just anchor the intestines. It carries blood vessels, lymphatics, and nerves to and from the intestines. On top of that, it stores fat as padding and releases serous fluid into the peritoneal cavity. This fluid helps reduce friction when intestines move.
Key Cell Types and Their Functions
Your small intestine’s remarkable functionality relies on five specialized cell types. Each type performs vital yet complementary functions in the intestinal epithelium.
Enterocytes and the brush border
Tall columnar cells called enterocytes make up most of the small intestine epithelium. These absorption powerhouses stand out because of their distinct apical structures—thousands of microvilli that create the brush border. This adaptation expands the surface area available to process and absorb nutrients. The enterocytes contain many membrane-bound enzymes, transporters, and channels needed for nutrient uptake. The microvilli serve as vesicle-generating organelles that release membrane components with host defense machinery into the intestinal lumen. The microvillous membrane works as a selective barrier that controls water and water-soluble substances while helping transport nutrients.
Goblet cells and mucus secretion
Goblet cells represent about 10% of all intestinal epithelial cells and produce protective mucus. These specialized exocrine glands have microvilli on their apical surface. They also feature a well-laid-out endoplasmic reticulum and Golgi apparatus needed for mucin production. The mucus they secrete creates a physical barrier that protects the epithelium from pathogens while supporting symbiotic bacteria. Transmembrane mucins are the foundations of this mucus layer. These mucins differ substantially from gel-forming mucins though they share the same gene nomenclature group (MUC). Goblet cells also release various interleukins and chemokines that affect immune responses.
Paneth cells and immune defense
Paneth cells sit at the base of intestinal crypts and play a vital role in innate immunity. They blend and release antimicrobial peptides—particularly α-defensins (cryptdins in mice, HD5 and HD6 in humans)—when bacteria stimulate them. These cells produce lysozyme, secretory phospholipase A2, and Reg3γ that work together to protect against pathogens. Paneth cells can sense enteric bacteria through MyD88-dependent toll-like receptor activation. Beyond their defensive role, they help create the stem cell niche by producing growth factors and Wnt signaling molecules.
Enteroendocrine cells and hormone release
Enteroendocrine cells make up just 1% of intestinal epithelial cells but form the body’s largest endocrine system. These cells detect luminal contents and release hormones that control digestion, motility, and metabolic processes. Scientists classify them into subtypes based on their main hormone secretions. These include I cells (cholecystokinin), S cells (secretin), K cells (glucose-dependent insulinotropic peptide), and enterochromaffin cells (serotonin). The cells create specialized structures called neuropods that link directly to the enteric nervous system and form neuroepithelial circuits.
Stem cells and epithelial renewal
Intestinal stem cells (ISCs) live within intestinal crypts and keep the epithelium constantly renewed. These cells show substantial plasticity and can self-renew and differentiate into all specialized intestinal cell types. The small intestine epithelium completely renews every 3-5 days as stem cells replenish enterocytes and goblet cells. Scientists have found multiple stem cell populations, including Lgr5+ cells at crypt bases and Fgfbp1+/Lgr4+ cells in the upper crypts. ISCs react to various signals, including those from Paneth cells that release growth factors, antimicrobial peptides, and Wnt ligands essential for stem cell maintenance.
Regional Differences in Histology
The small intestine has evolved into a specialized tube with distinct histological features in each section that optimize its functions.
Duodenum: Brunner’s glands and neutralization
Unique Brunner’s glands in the duodenum’s submucosa secrete alkaline mucus containing bicarbonate. These compound tubular glands concentrate heavily in the proximal few centimeters between the pylorus and entry points of the bile and pancreatic ducts. The glands pack the duodenal submucosa so densely that they often hide the typical submucosal connective tissue. Their main goal involves neutralizing acidic chyme from the stomach to create an optimal pH environment for intestinal enzymes. Secretin, vagal signals, and direct stimulation trigger these secretions, while sympathetic activation inhibits them. The duodenum’s structure shows flatter villi and fewer plicae compared to the jejunum.
Jejunum: longest villi and absorption
The jejunum features remarkably long, finger-like villi that maximize its absorptive capacity. The capillaries in jejunal villi can absorb nutrients at rates that are a big deal as it means that they exceed other organs by several hundred times per gram of tissue, including brain and contracting skeletal muscle. Rich blood vessels give the jejunum its distinctive red appearance. Nutrient absorption reaches peak efficiency here—normal human subjects’ perfused jejunal segments can absorb glucose at rates exceeding 4000 μmol h−1 per centimeter. A unique microscopic architecture creates widened intercellular channels during absorption that allow concentrated nutrients to move quickly to capillaries without significant dilution.
Ileum: Peyer’s patches and immune surveillance
Peyer’s patches, which are the foundations of the ileum’s structure, consist of hosted lymphoid follicles in the lamina propria and submucosa. The distal 25 cm of the ileum contains almost 50% of these immune structures. The patches’ specialized M cells (microfold cells) in their follicle-associated epithelium transport luminal antigens to underlying immune cells. These M cells work differently from typical enterocytes as they lack microvilli, which allows them to sample intestinal contents. Peyer’s patches act as immune sensors that recognize potential risks while developing tolerance to food antigens. The ileum’s enhanced protection comes from its higher proportion of goblet cells compared to the duodenum or jejunum, which boost mucus production.
Clinical Relevance and Pathological Changes
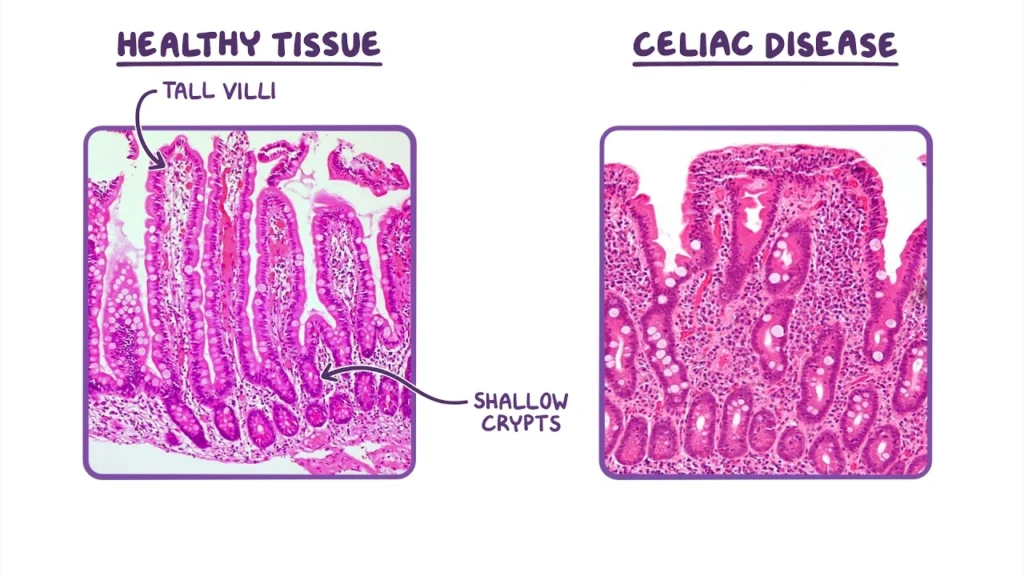
Pathological changes in small intestine histology can dramatically change digestion, absorption, and barrier functions. These changes explain many clinical conditions and their symptoms.
Celiac disease and villous atrophy
Celiac disease affects about 1% of the population as an autoimmune condition, yet doctors rarely diagnose it. Your immune system launches an uncontrolled response that damages the small intestine’s epithelium at the time you consume gluten. The disease’s key histological feature is villous atrophy—intestinal villi flatten while crypts become hyperplastic. This structural damage reduces the absorptive surface area significantly and leads to symptoms like diarrhea, bloating, and malnutrition.
Doctors need both serology and biopsy to diagnose celiac disease. A small intestine biopsy shows characteristic villi flattening and increased intraepithelial lymphocytes. About 40% of patients still demonstrate persistent villous atrophy even on a gluten-free diet. Several medications—including PPIs, NSAIDs, and SSRIs—associate with ongoing intestinal damage.
Cholera and epithelial barrier breakdown
Vibrio cholerae produces Cholera toxin (CT), which causes severe watery diarrhea through two mechanisms. Scientists originally thought CT’s pathology only increased chloride secretion, but new findings show it also disrupts the epithelial barrier. CT’s enzymatic A-subunit (CtxA) enters intestinal cells and increases cAMP after binding to GM1 ganglioside receptors.
The elevated cAMP activates chloride channels and blocks Rab11/exocyst-mediated protein trafficking to cell junctions. E-cadherin and other vital junctional proteins become misplaced, creating visible gaps between cells under electron microscopy. Fluid leaks through these structural defects in the paracellular pathway, which makes the diarrhea worse beyond active ion secretion.
Radiation and chemotherapy effects on crypts
The intestinal epithelium’s rapid turnover makes it vulnerable to treatments targeting dividing cells. Gastrointestinal toxicity affects 40-100% of patients receiving chemotherapy and radiation therapy. These treatments damage crypt stem cells first, which leads to widespread crypt cell death.
The damage shows up as crypt loss, villus atrophy, and compromised barrier function. DNA strand breaks and reactive oxygen species form first, followed by pro-apoptotic proteins and inflammatory pathways. Radiation injury also depletes intestinal trefoil factor (ITF), a compound that helps epithelial cell migration and tissue repair. Regenerating crypts often show irregular shapes and displaced Paneth cells after radiation.
Vitamin B12 malabsorption in ileum resection
The terminal ileum absorbs vitamin B12 exclusively, so ileal resection can cause B12 deficiency. B12 absorption depends directly on the length of remaining terminal ileum after surgery. Patients usually maintain normal B12 absorption with less than 20 cm of terminal ileum removed. The risk of abnormal B12 absorption rises to 50% with 20-60 cm removal, while losing more than 60 cm almost always causes malabsorption.
Doctors can monitor for deficiency, give supplements, or test absorption capacity in patients with moderate resections (20-60 cm). The body maintains B12 absorption even when surgeons remove other segments, as long as they preserve the terminal ileum.
Conclusion
The small intestine’s structure shows amazing adaptations that match its main functions of digestion and absorption. Its specialized features, from finger-like villi to the tiny brush border, expand the absorptive surface area by a lot. This creates a biological wonder of efficiency.
Different parts of the small intestine show unique adaptations for specific roles. The duodenum has special Brunner’s glands that neutralize stomach acid. The jejunum shows the longest villi to absorb nutrients better. The ileum contains many Peyer’s patches that guard the immune system.
The small intestine’s cells improve its function in different ways. Enterocytes absorb nutrients and goblet cells make protective mucus. Paneth cells fight against pathogens while enteroendocrine cells control digestion by releasing hormones. Stem cells at the base of crypts help renew tissue, which lets the whole epithelium grow back every 3-5 days.
Knowledge of these tissue features explains many clinical conditions. Celiac disease hurts the villi and cholera breaks epithelial barriers. Radiation therapy damages fast-growing crypt cells and removal of the ileum stops vitamin B12 absorption. These links between microscopic structure and clinical signs show why doctors need to understand tissue structure.
Your small intestine is more than just long with great absorption power. It demonstrates how form and function work together perfectly. Each cell, layer, and regional specialty helps process nutrients while protecting your body from the outside world. This delicate balance, when upset, leads to various diseases that affect millions of people worldwide.
Key Takeaways
Understanding small intestine histology reveals how microscopic structure directly supports digestive function and explains common clinical conditions.
• The small intestine features four specialized layers with villi and crypts that maximize absorptive surface area by up to 600-fold through finger-like projections and microvilli brush borders.
• Five key cell types work together: enterocytes absorb nutrients, goblet cells secrete protective mucus, Paneth cells provide immune defense, enteroendocrine cells release hormones, and stem cells renew the epithelium every 3-5 days.
• Each region has distinct specializations – duodenum neutralizes stomach acid with Brunner’s glands, jejunum maximizes absorption with longest villi, and ileum provides immune surveillance through Peyer’s patches.
• Pathological changes directly correlate with clinical symptoms: celiac disease flattens villi causing malabsorption, cholera disrupts epithelial barriers causing diarrhea, and radiation damages rapidly dividing crypt cells.
• The terminal ileum uniquely absorbs vitamin B12, making resections beyond 60cm almost guarantee deficiency, while shorter resections may preserve normal absorption capacity.
This intricate histological organization demonstrates how cellular architecture directly enables the small intestine’s remarkable ability to absorb 90% of nutrients while maintaining protective barrier functions.
FAQs
Q1. What are the main functions of the small intestine?
The small intestine plays a crucial role in digestion and absorption of nutrients. It breaks down food, absorbs about 90% of nutrients and water, and moves food along the digestive tract. Each section (duodenum, jejunum, and ileum) has specialized functions for optimal nutrient processing and absorption.
Q2. How does the structure of the small intestine support its function?
The small intestine has a highly specialized structure to maximize its absorptive capacity. It features finger-like projections called villi and microvilli (brush border) that dramatically increase the surface area for nutrient absorption. This unique architecture allows the small intestine to efficiently process and absorb nutrients.
Q3. What are the key cell types in the small intestine and their roles?
The small intestine contains several specialized cell types: enterocytes for nutrient absorption, goblet cells for mucus secretion, Paneth cells for immune defense, enteroendocrine cells for hormone release, and stem cells for continuous epithelial renewal. Each cell type plays a crucial role in maintaining intestinal function and health.
Q4. How do pathological changes in the small intestine affect its function?
Pathological changes can significantly impact small intestine function. For example, celiac disease causes villous atrophy, reducing the absorptive surface area and leading to malabsorption. Cholera toxin disrupts the epithelial barrier, causing severe diarrhea. Understanding these histological changes helps explain the symptoms and complications of various intestinal disorders.
Q5. Why is the terminal ileum important for vitamin B12 absorption?
The terminal ileum is uniquely responsible for vitamin B12 absorption. Resection of this area can lead to B12 deficiency, with the risk increasing based on the length of ileum removed. Preserving at least 20 cm of terminal ileum is crucial for maintaining normal B12 absorption capacity.